Gunze products currently
available outside of the U.S.
Proven, Trusted Medical Products
Gunze manufactures a number of bioabsorbable materials, devices, and healthcare products that have not yet been cleared or approved for use in the U.S. These items are used, however, with the full trust and confidence of surgeons in hospitals around the world and could be made available to U.S. surgeons in the future.
Our portfolio includes:
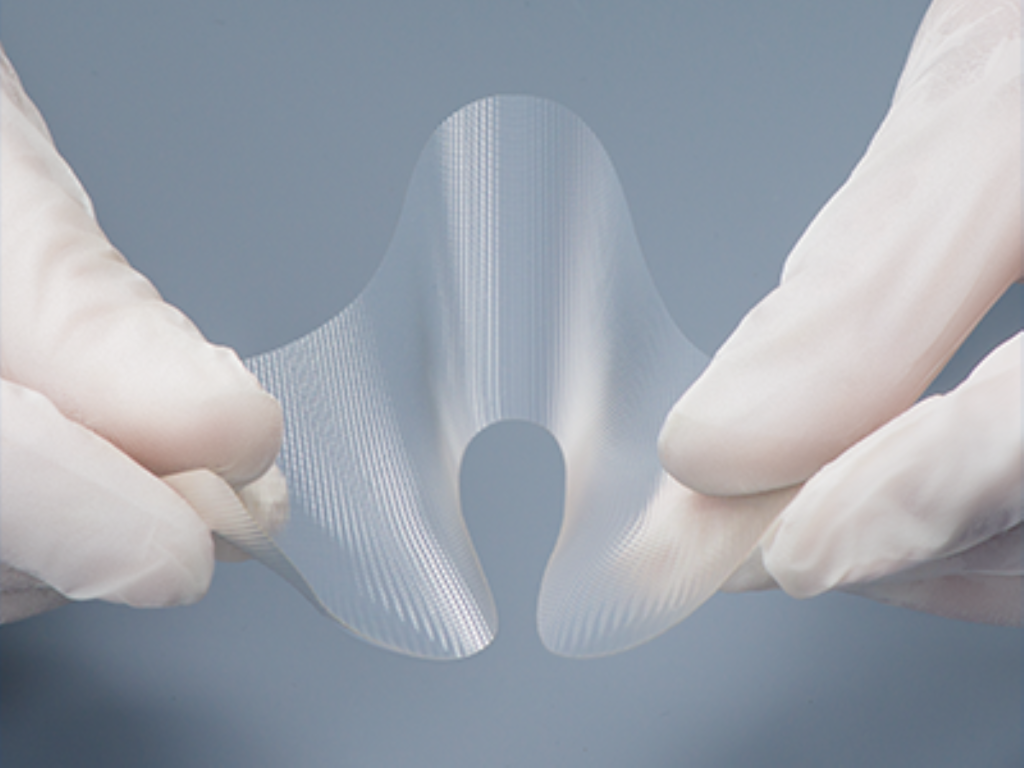
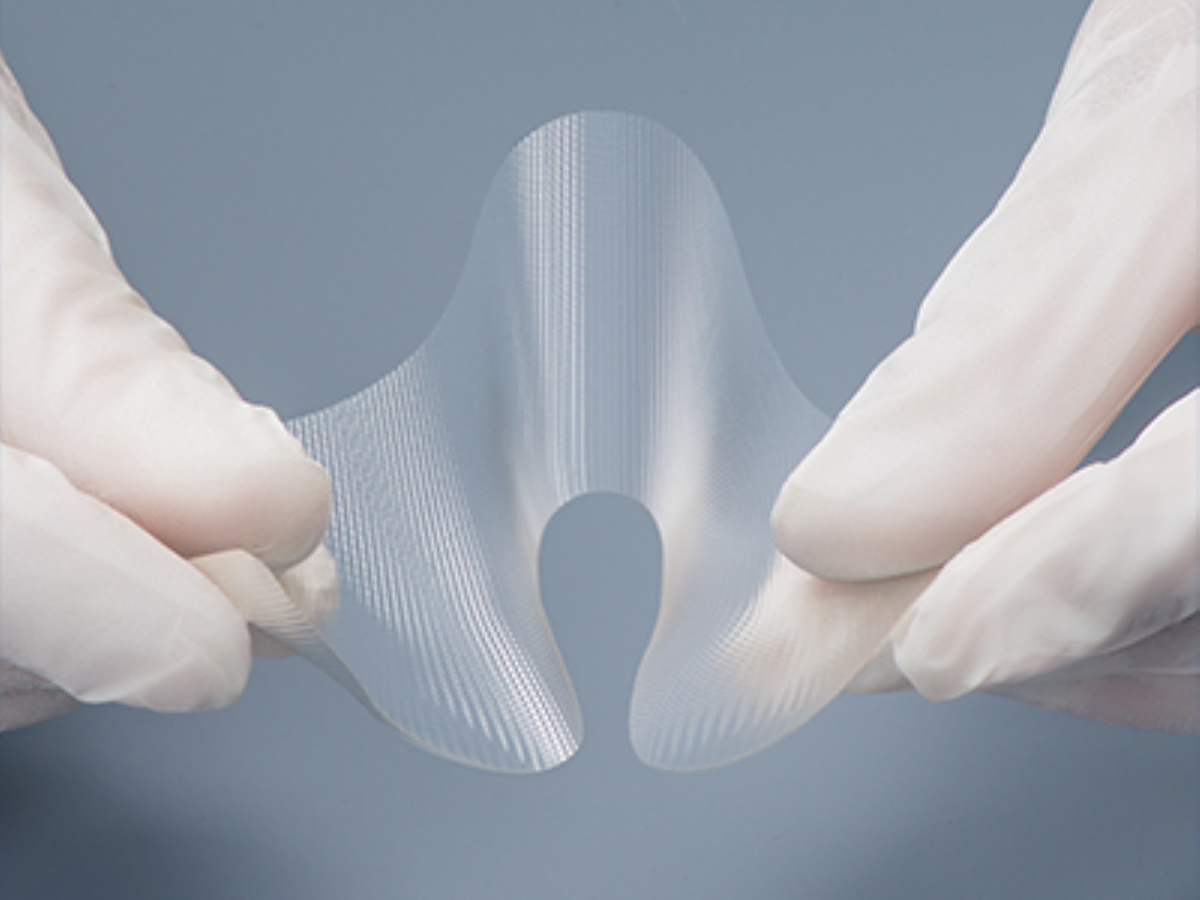
Absorbable Adhesion Barrier
Gunze’s absorbable adhesion barrier is an embossed, flexible, and deployable film adhesion barrier composed of gelatin. This product reduces the incidence, extent, and severity of postoperative adhesions and is degraded enzymatically in the body.
Scaffold for Cartilage Regeneration
Gunze’s bioabsorbable nonwoven felt is used for the treatment of traumatic full-thickness, chondral, and osteochondral defect. Consisting of 100 percent PGA, the felt’s porous, fibrous structure acts as a shape and guidance template for cartilage-like tissue development.
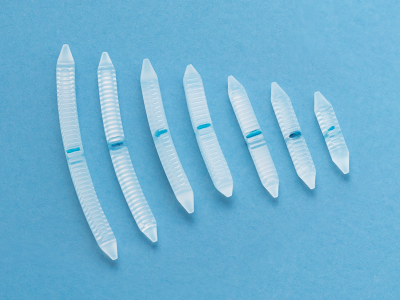
Dura Substitutes Single Layer (PGA)
Gunze’s absorbable single layer dura substitute is made of polyglycolic acid (PGA) non-woven fabric and gradually degrades by hydrolysis. This product is used as a prosthetic material for dura mater defects and enables surgeons to perform the procedure without suture.
Dura Substitutes Multi-Layered
Gunze’s absorbable multi-layered dura substitute is made of poly(L-lactic acid/ε-caprolactone) copolymer P(LA/CL) film and layered with PGA felt to provide strength, elasticity, and a leak-resistant seal. The three-layered structure can effectively support sutured areas.
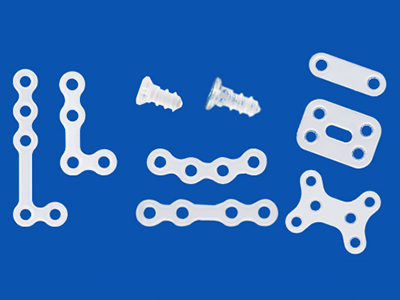
Absorbable Bone Fixation Device
Gunze’s absorbable bone fixation device is made of poly-L-lactic acid (PLLA) that gradually degrades upon hydrolysis and is absorbed in the human body. Gunze manufactures different versions of this bone fixation device for CMF, trauma, and rib and sternum applications.
Visit Gunze’s corporate website to learn more about bioabsorbable materials, devices, and healthcare products that are used in other parts of the world but not yet cleared or approved for use in the U.S.